Alzheimer's Clinical Trials Consortium
Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) Study and Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by progressive decline in cognitive function and the ability to perform activities of daily living, ultimately resulting in dementia with fatal complications.
The amyloid hypothesis of AD postulates that the accumulation of amyloid-β peptide (Aβ) is an early and necessary event in the pathogenesis of AD. This hypothesis suggests that treatments that slow the accumulation of Aβ in the brain or increase clearance of Aβ may be able to slow the progression of the AD clinical syndrome.
Converging evidence from both genetic at-risk and age at-risk cohorts suggests that the pathophysiological process of AD begins well more than a decade before the clinical stage now recognized as AD dementia, and that neurodegeneration is already apparent by the stage of mild cognitive impairment (MCI)/prodromal AD. Recent clinical trial results in mild-to-moderate AD dementia, as well as evidence from transgenic animal experiments, suggest that earlier intervention may be most beneficial to fully impact the clinical progression of the disease, particularly with therapies targeted at Aβ reduction.
Data from autopsy cohorts and cerebrospinal fluid (CSF) and positron emission tomography (PET) scan amyloid imaging studies demonstrate that approximately 30% of individuals over the age of 65 have evidence of amyloid pathology. Clinically “normal” older individuals with biomarker evidence of elevated amyloid pathology demonstrate AD-like abnormalities on functional and structural imaging, perform less well on cognitive tests compared to amyloid-negative older individuals, are more likely to report subjective memory concerns, and are at increased risk for cognitive decline and progression to MCI and AD. Recent academic consensus workgroups focused on developing guidelines for earlier diagnosis of AD and research diagnostic criteria have defined a stage of “preclinical AD,” based on biomarker evidence of AD pathology before the stage of clinically evident symptoms of cognitive impairment.
The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease “A4” study was a Phase 3, double-blind, placebo-controlled study in subjects with preclinical AD. The A4 trial preclinical AD study population was defined as subjects with evidence of brain amyloid pathology on PET amyloid imaging who were still clinically normal but at high risk for cognitive decline. The A4 study was designed to test the hypothesis that solanezumab, a monoclonal antibody that binds Aβ, would slow decline on a cognitive composite measure designed to be sensitive to early decline in the preclinical stages of AD. This study collected additional data on the long-term safety and efficacy of solanezumab, including computerized cognitive outcomes, as well as self and study partner reports of daily-life cognitive function outcomes, a brief resource utilization inventory, and biomarker and imaging measures.
Learn more about our studies
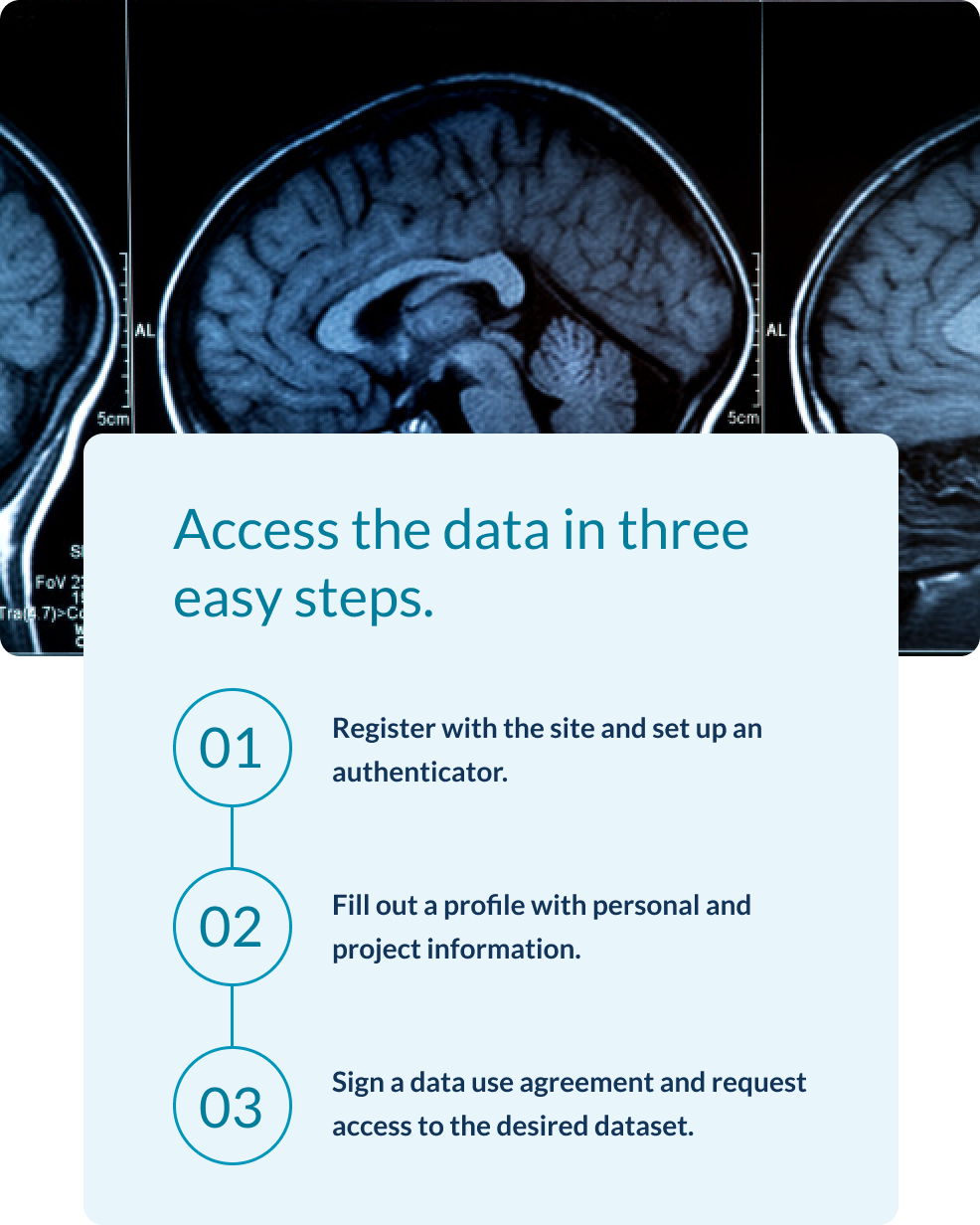
The A4/LEARN screening dataset is the largest cohort of cognitively unimpaired older individuals with amyloid imaging and detailed cognitive testing available to date and has been requested by over 1500 investigators from around the world.
Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) Study
A4 was fully enrolled with 1169 randomized participants across 67 sites in 2017. The participants were cognitively unimpaired individuals between 65 and 85 years of age with evidence of elevated amyloid plaque accumulation in the brain on screening PET.
Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study
The LEARN Study was funded by the Alzheimer’s Association and GHR Foundation and enrolled 540 clinically unimpaired individuals who screen-failed for the A4 Study on the basis of not showing elevated amyloid on screening amyloid PET imaging, followed longitudinally with all of the same assessments.
The story
A4 was the first multicenter trial in sporadic preclinical (pre-symptomatic) Alzheimer’s disease (AD) and tested whether the drug, solanezumab, could slow the progression of cognitive decline related to AD in individuals who did not yet show clinical symptoms.
The concept for this project arose from analyses of longitudinal data from multiple observational cohorts, that indicated that amyloid elevation in brain in clinically normal individuals is associated with subtle cognitive and biomarker evidence of decline. Thus ‘preclinical AD’ may be the optimal stage at which to intervene with anti- amyloid therapies, with sensitive cognitive tests as well as secondary biomarker measurements as outcomes to establish efficacy.
Partnerships and funders
Solanezumab, a monoclonal antibody against monomeric amyloid peptide, which was selected as the therapeutic, and a public private partnership with National Institute on Aging (NIA) at the National Institutes of Health (NIH) and Eli Lilly and Co. was established to carry out the trial, with additional funding contributed by philanthropic groups, and in-kind support from multiple partners.
In a first for a Phase 3 trial, A4 pre-randomization data and LEARN data were publicly released within one year after completion of randomization.
We are very pleased to share the full longitudinal A4 and LEARN dataset, and hope that these data will serve to accelerate future research to prevent Alzheimer’s disease dementia.
Results
The A4 Study clinical trial results were announced in March 2023, and published in the New England Journal of Medicine in July 2023. Very disappointingly, no beneficial treatment effects were observed with solanezumab compared to placebo, potentially because solanezumab only modestly slowed amyloid accumulation, and did not reduce amyloid PET below baseline levels. The strongest predictors of cognitive decline in A4 were higher baseline amyloid PET, plasma markers of elevated amyloid and tau, and neocortical tau PET in a subset. These findings suggest that higher levels of Alzheimer’s biomarkers at baseline are associated with high risk of cognitive decline, and that aggressive amyloid reduction may be necessary even at the stage of preclinical AD.
We wish to express immense gratitude to the very dedicated A4 and LEARN participants and their study partners, and to the large A4 and LEARN Study teams across many institutions.
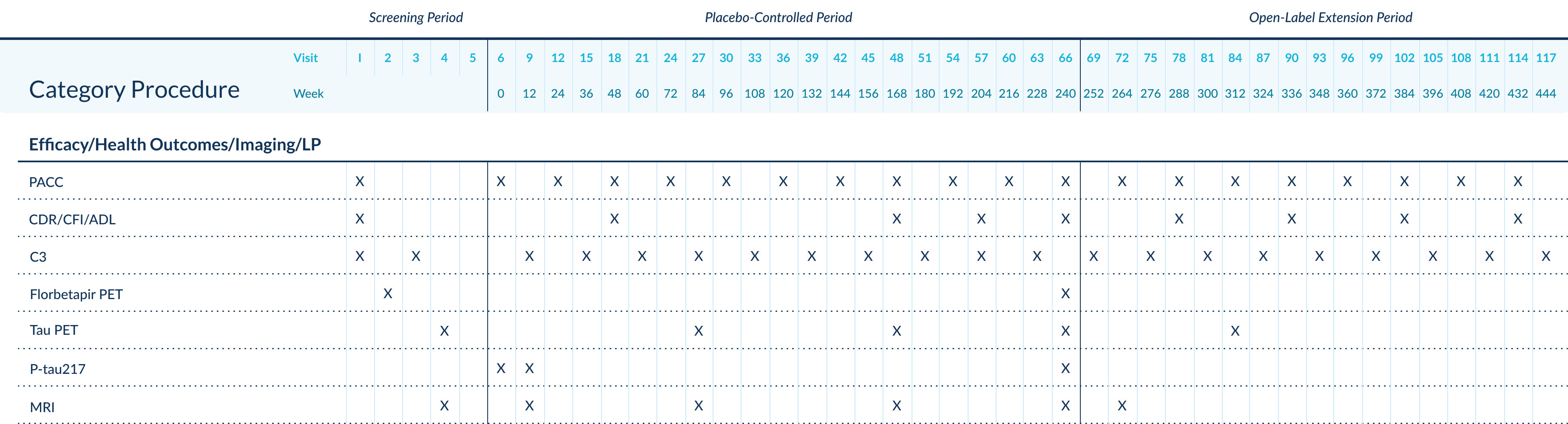
Study Schedule, A4 Protocol
Discover the latest in research
March 29, 2022
Associations of stages of objective memory impairment with amyloid PET and structural MRI: The A4 study
August 17, 2021
Feasibility study for detection of retinal amyloid in clinical trials: The Anti‐Amyloid treatment in asymptomatic Alzheimer's disease (A4) trial
June 1, 2022
Divergent cortical tau positron emission tomography patterns among patients with preclinical Alzheimer disease
Frequently asked questions
For additional information, please contact us.
For general inquiries, please contact us. For data requests, kindly .

Alzheimer's Clinical Trials Consortium
The A4 and LEARN Study is affiliated with Alzheimer's Clinical Trials Consortium. ACTC’s mission is to provide an optimal infrastructure, utilizing centralized resources and shared expertise, to accelerate the development of effective interventions for Alzheimer’s disease and related disorders.

